|
 Chlorovibrissea phialophora Chlorovibrissea phialophora
BiostatusPresent in region - Indigenous. Endemic
Images (click to enlarge)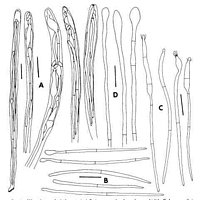
Caption: Fig. 4. Chlorovibrissea phialophora. A, Asci. B, Ascospores that do not have a phialide. C, Ascospores that do have a phialide. D, Paraphyses. All drawn from holotype. Scale bars = 10 µm | 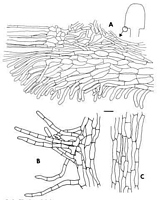
Caption: Fig. 3. Chlorovibrissea phialophora. A, Margin with portion of hymenium (on left) and ectal excipulum (on right). B, Hairs from surface of stipe. C, Medullary tissue of stipe. All drawn from the holotype. Scale bar = 10 µm. | 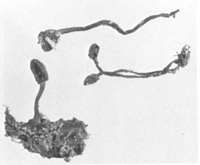
Caption: Fig. 2. Chlorovibrissea phialophora, ascomata. X 1, approx. | 
Owner: B. Hartley | 
Caption: Fruiting bodies up to about 10 mm tall
Owner: B. Hartley, DOC |
Article: Kohn, L.M. (1989). Chlorovibrissea (Helotiales, Leotiaceae), a new genus of austral discomycetes. Memoirs of the New York Botanical Garden 49: 112-118.
Description: Ascomatal Morphology. Apothecia 10-30 mm high, gregarious, stipitate-capitate, produced
from a green, pulvinate, mycelial pad 1.5-2.0 mm diam. on the surface of nonblackened
wood; stipe 10-25 mm long x 1 mm diam., green, villose or glabrous, longitudinally or
spirally furrowed, circular in section; cap subglobose to cylindrical, 2-7 mm high x 2-5 mm
wide, dark green to nearly black, smooth, viscid or appearing gelatinous at maturity,
separated from stipe by a distinct groove; groove not apparent at maturity; ascomata leaching
an olivaceous pigment in 10% KOH. Ascomatal Anatomy. Asci 8-spored, (100-) 105-116(-123) x 5-6 µm, produced from croziers, narrowly clavate to cylindrical, tapering toward the
base and there forming a small foot; apical ring intensely J + in Melzer's reagent, with a
"crown" of intense staining in methyl blue or phloxine just below apical ring; ascospores
fillings, each ascus. Ascospores (35.0-) 42.0-51.6 (-60.0) x 1.0-1.5 µm multiseriate, hyaline;
filiform, tapering slightly from rounded apex to pointed base, 0-1-septate; apical cell
enlarging while still in ascus and forming a subglobose to cylindrical phialide, collarette
cupulate, ca. 1.5 µm deep; length of phialide from apex to first septum of ascospore (9-)10-17(-20) µm; spore eventually elongating to 85 µm, all cells of spore enlarging, phialide
arising only from apical cell. Ascoconidia arising from phialides, 1.7-4.0(-5.0) x 1.0-1.5 µm,
oblong, lacking a basal abscission scar, eguttulate, hyaline. Paraphyses extending beyond
asci by ca. 10 µm, hyaline, unbranched, not anastomosing, filiform, 2-3 µm broad, Septate,
apical cell ca. 40 µm long, subsequent cells ca. 20 µm long, tip clavate to subglobose, 4-5
µm. Subhymenium poorly developed, consisting of tightly interwoven, narrow,
nonpigmented hyphae. Medullary excipulum composed of textura intricata, hyphae 3-4 µm
broad with walls 0.5 µm thick, becoming gelatinized at maturity, continuous with medulla of
stipe; zone adjacent to subhymenium turning green in Melzer's reagent. Ectal excipulum
poorly developed, forming a thin, ca. 80 µm wide layer on underside of cap, composed of
prosenchyma with the long axis of cells parallel to the excipular surface, cells 25-35 µm long
x ca. 8 µm broad, walls 1.0-1.5 µm thick, cells producing hyphal hairs up to 30 µm long x
surface cells producing hyphal hairs up to 30 µm long x 4-5 µm broad, septate, pale green;
ectal excipulum continuing down stipe for a distance of ca. 200 µm. Stipe lacking a well-developed cortex, hyphae continuous with medullary excipulum, with refractive, gelatinized
walls; stipe hollow at maturity; cells at surface of stipe 6-8 µm, somewhat broader than those
within, producing hyphal hairs, hairs 25-35 µm long x ca. 8 µm broad, septate, infrequently
branched, pale green, forming a fine tomentum over surface of stipe, absent at maturity.
Mycelial pad from which stipe arises composed of interwoven, septate, branched, green
hyphae 3-4 µm broad and to 100 µm long, with many free ends giving pad a wooly aspect.
Notes: Chlorovibrissea phialophora differs from the other three species, C tasmanica, C melanochlora, and C. bicolor, in producing ascoconidia and in turning green in a narrow zone of the medullary excipulum adjacent to the subhymenium. Chlorovibrissea tasmanica has narrower paraphysis apices, narrower ectal excipular cells, and a more luxuriant tomentum on the stipe composed of longer hyphal hair than in C. phialophora. Chlorovibrissea melanochlora produces longer ascospores, asci, and stipe hairs, and narrower ectal excipular cells. Chlorovibrissea bicolor produces longer ascospores which are apically coiled in the ascus, narrower paraphysis apices, narrower ectal excipular cells, and longer hyphal hairs.
The formation of ascoconidia is not uncommon in the Helotiales. Species of Geoglossum Pers.: Fr., Cudonia Fr., Spathularia Pers. (Berthet, 1964), Ascocoryne Groves & Wilson (Roll-Hansen & Roll-Hansen, 1979), Tympanis Tode: Fr. (Groves, 1952), and Rutstroemia Karsten (White, 1941) among others, are known to produce conidia from ascospores still in the ascus. Conidiogenesis in C. phialophora is unusual because the tip of the ascospore is converted into a more or less complex phialide. In the other genera, conidia are produced laterally or terminally on barely differentiated conidiogenous loci. Discharged ascospores of Encoelia pruinosa (Ell. & Everh.) Torkelson & Eckblad sometimes produce phialidic openings through which microconidia develop (Juzwik & Hinds, 1984), and phialides arise directly from the surface of discharged ascospores of some species of Rutstroemia (White, 1941).
|