|
 Cortinarius rubrocastaneus Cortinarius rubrocastaneus
SynonymsGymnopilus rubrocastaneus
BiostatusPresent in region - Indigenous. Endemic
Images (click to enlarge)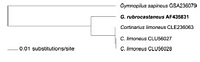
Caption: Fig. 2 Dendrogram resulting from a cluster analysis (UPGMA) of the alignment
shown in Fig. 1. | 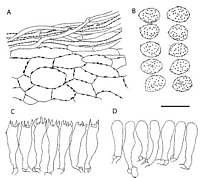
Caption: Fig. 3 Cortinarius rubrocastaneus (drawn from a fragment of the isotype
labelled KSBR 130). A, pileipellis; B,basidiospores;
C, basidia; D, marginal cells. Sc | 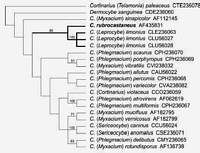
Caption: Fig. 4 Strict consensus of four equally parsimonious trees of length 529; consistency index =
0.501; retention index = 0.457; rescaled consistency index = 0.229. Bootstrap values (%) from
1000 replicate analyses are shown above the bran | 
Owner: J.A. Cooper | 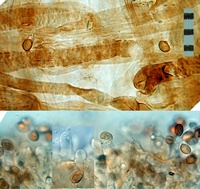
Caption: Top: cap hyphae: Bottom: cheilocystidia (KOH)
Owner: J.A. Cooper | 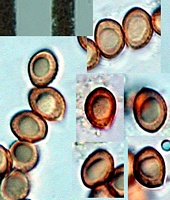
Caption: spores
Owner: J.A. Cooper | 
Owner: J.A. Cooper | 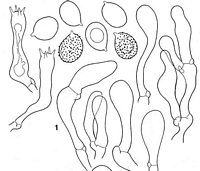
Caption: (1) Gymnopilus rubrocastaneus,basides, cellules marginales et spores. | |
Article: Soop, K. (2001). Contribution à l'étude de la mycoflore cortinarioïde de Nouvelle-Zélande. Bulletin Trimestriel de la Société Mycologique de France 117(2): 91-132.
Description: Chapeau 2-3,5 cm, sec, peu hygrophane, obtusément conique puis convexe à
campanulé, rouge de palissandre saturé à rouge foncé, glabre; marginelle jaune à l'état
jeune, puis legérèment cannelée. Lames jaune moutarde à orangé au début, largement
émarginées, moyennement distantes (L = 36,1= 1-2), arête concolore. Stipe assez
robuste, 4-7 x 0,6-0,9 cm, cylindrique à atténué vers le bas, jaune-brun foncé avec des
fibrilles rouge orangé à bran jaune, apex plus pâle. Voile orangé brunâtre, fugace;
cortine presque blanche, assez abondante. Chair jaune brun foncé, odeur et saveur
nulles, fluorescence nulle.
Réactions chimiques : réaction à la soude fortement brun-rouge à rouge sur le voile du
stipe, brun-rouge à banale ailleurs; réaction nulle au gaïac.
Spores subglobuleuses, 6-7,5 x 5,2-6 µm, peu verruqueuses; basides à 4 stérigmates,
longueur autour de 30 µm; cellules stériles nombreuses, clavées, 25-35 x 8-12 µm,
non différenciées, certaines contenant un pigment jaune brunâtre, parfois en sphérules;
boucles présentes.
Habitat: Écologie : sur les racines de Nothofagus, fasciculé, peu commun.
Notes: Étymologie : de Tuber, « rouge », et castanea, « châtaigne », dû à la coloration
générale.
On reconnaît cette espèce à son chapeau rouge palissandre et à sa croissance
fasciculée, ce qui n'est pas sans rappeler certains Hypholoma. Contrairement à la
plupart des Gymnopilus, le goût n'est pas amer. Flammula purpurata Cleland (1934)
lui ressemble, mais le coloris est plus pourpré rougeâtre avec une teinte verte.
Article: Orlovich, D.A.; Oliver, A.-M.B. (2002). The taxonomic identity of Gymnopilus rubrocastaneus recently described from New Zealand. New Zealand Journal of Botany 40(3): 481-487 (http://www.rsnz.org/publish/abstracts.php).
Description: The species is described in detail as Gymnopilus rubrocastaneus in Soop (1999, 2001). We
provide here a description of microscopic characters measured from the fragment of isotype
material that was used here for DNA analysis.
DESCRIPTION: Basidiospores (20/1) L x W x B = 6.4-7.6 ( µx = 7.1) x 5.2-5.6 ( µx = 5.5) x
4.8-6.4 ( µx = 5.5)µm, L/W = 1.3, L/B = 1.3, broadly ellipsoid subglobose,pale yellow with
areas golden, weakly dextrinoid, ornamentation fine but surface well covered, dark, plage
absent. Basidia (10/1) L x W =27.2-32.8 ( µx = 30.1) x 5.6-8.8 ( µx = 6.8) µm
with sterigmata up to 4.8 µm long, 4-spored, clavate,hyaline or golden yellow. Marginal cells
(10/1) L x W = 24.8-32.8 ( µx = 28.9) x 6.4-8 ( µx = 7.3) µm,clavate, hyaline, similar to
basidioles, which were slightly smaller and often contained golden yellow pigment. Clamp
connections present. Pileipellis acutis of hyaline to yellow-pigmented hyphae, some encrusted
with rust brown ornamentation (6/1) W =4-6 ( µx = 5.7) µm.
Notes: DNA sequencing and cluster analysis
Alignment of the ITS sequence obtained from Gymnopilus rubrocastaneus with three
Cortinarius limonius sequences and one Gymnopilus sapineus sequence (Fig. 1) shows that
the ITS sequence of G. rubrocastaneus is more similar to C. limonius than to G. sapineus.
Sequence dissimilarities were 4.81-4.93% between Gymnopilus rubrocastaneus and
C.limonius, and 23.04% between G. rubrocastaneus and G. sapineus. Dissimilarities between
the three C. limonius sequences were 0.00-0.15% and thesewere 20.18-20.25% dissimilar to
G. sapineus. The cluster analysis produced a rooted dendrogram (Fig.2) that illustrates the
high degree of similarity between G. rubrocastaneus and C. limonius.However, the sequences
for those two species are not identical, indicating that there is some genetic difference
between the taxa.
Relationship to Gymnopilus
Gymnopilus rubrocastaneus possesses a webby cortina rather than a membranous, fibrillose,
or absent partial veil typical of other Gymnopilus. The mahogany-red, hygrophanous pileus
does not resemble the typical golden, non-hygrophanous pilei typical of Gymnopilus (or when red
colorationis present in Gymnopilus, the pileus is still not hygrophanous). Unlike most
Gymnopilus, the taste of the cap flesh is not bitter (Soop 2001). The marginal cells (described
as sterile cells by Soop 2001) are clavate as opposed to the fusoid ventricose, frequently
capitate cheilocystidia present in other Gymnopilus. The basidiocarps are recorded as occurring
"sur les racines de Nothofagus" (Soop 2001), suggestive of a close association with
the ectomycorrhizal tree Nothofagus, whereas mostGymnopilus species are found on dead
wood.
NOTES: This species is similar to C. limonius in having an hygrophanous pileus with a
yellow margin, a yellow cortina, yellow-orange lamellae, broadly elliptical to subglobose
spores, clavate basidia, and marginal cells that resemble the basidioles. The species differs
from C. limonius in having a fasciculate growth habit, a darker chestnutred pileus, smaller
basidiospores, and more slender basidia and marginal cells.
PHYLOGENETIC ANALYSISA cladistic analysis of 22 taxa and 170
informative characters found 4 equally parsimonious trees of length 529 (Fig. 4). Bootstrap
analysis clearly supported the monophyly of Cortinarius rubrocastaneusand C. limonius
(subgenus Leprocybe). Other pairs of species well supported by bootstrap analysis (>70%)
were C. atrovirens Kalchbr. and C.multiformis (Pers.:Secr.) Fr.
(subgenus Phlegmacium),C.
mucifluus Fr. and C. vernicosus Seidl (subgenus Myxacium), and
C. caninus (Fr.) Fr. andC.
anomalus (Fr.:Fr.) Fr. (subgenus Sericeocybe).Dermocybe sanguinea Wulf.:Fr. was found to
bea sister to the remaining Cortinarius species (excluding the outgroup subgenus Telamonia)
in all equally parsimonious trees but there was no bootstrap support for this or other deep
branches in the tree.
DISCUSSION
Morphological and molecular analyses of type material of Gymnopilus rubrocastaneus
indicate a close relationship to Cortinarius (Leprocybe) limonius and a relatively distant
relationship to Gymnopilus, thus prompting our combination to C.rubrocastaneus. The
morphological characters of a smooth, dark red pileus, the yellow cortina, and broadly
ellipsoid-subglobose spores all fit within the concept of Cortinarius subgenus Leprocybe
section Limonei Moser and this is further supported by the close genetic relationship to C.
limonius, the type species of that section (Singer 1986). The lack of yellow fluorescence in C.
rubrocastaneus may be a concentration effect and should be assessed in further collections,
although fluorescence is also reported to be weak in C. limonius (Moser 1983).
Cortinarius limonius is not recorded in New Zealand, thus, this clade indicates a close
relationship between a Northern and a Southern Hemisphere species. One other New Zealand
species in Cortinarius subgenus Leprocybe is C. pholiotellus Soop that has strong fluorescence
(K. Soop pers. comm.) and is thus not referred to Leprocybe section Limonei. The lack of
support for lower branches of the phylogenetic tree presented here is not surprising given our
relatively conservative treatment of gaps as missing data in the analysis.
Insertion/deletion (indel) characters were found by Høiland & Holst-Jensen (2000) to be
phylogenetically informative and necessary to resolve deeper branches in their phylogenetic
analysis of Cortinarius. We found that most gaps in our alignment were due to
length polymorphisms of T-rich regions of the sequences that, when combined with the small
number of representative taxa from each subgenus, led to difficulty coding the gaps with
confidence. The monophyly of subgenus Leprocybe has been questioned by previous authors.
Cortinarius limonius was shown by Liu et al. (1997) to be related to C. vibratilis (Fr.) Fr.
(subgenus Myxacium) but this relationship was poorly supported (52% bootstrap). This
relationship was not supported by the analysis of Høiland & Holst-Jensen (2000),although the
two sequences used in those studies (GenBank Høiland: CVI238032; Liu: CVU56033) are not
the same and we suggest that they probably represent different taxa. Seidl (2000) found
a relationship between C. limonius and C. allutus Fr.487 Orlovich & Oliver -Cortinarius
rubrocastaneus comb. nov(subgenus Phlegmacium) but again this had only 57% support from
a bootstrap analysis. OtherLeprocybe species have been shown to be related to Cortinarius
species in other subgenera, with C. gentilis (Fr.) Fr. having a close relationship to members of
subgenus Telamonia (Liu et al. 1997; Høiland & Holst-Jensen 2000) and C. rubellusCooke
being a sister species to Dermocybe (Høiland& Holst-Jensen 2000). Neither species appears
to be closely related to C. limonius and C. rubrocastaneus. The present discovery of a close
relationship between C. rubrocastaneus and C. limonius is the first report of a well-supported
monophyletic group within the subgenus Leprocybe sens. lat. The monophyly of the subgenus
remains unresolved.Cortinarius subgenus Leprocybe sens. lat. contains five sections:
Leprocybe, Raphanoidei, Bolares, Orellani, and Limonei (Moser 1983). With the discovery
that C. limonius is not closely related to C. gentilis (Liu et al. 1997;
Høiland & Holst-Jensen 2000), section Limonei has already been shown to be not monophyletic. Phylogenetic
analyses including other Cortinarius subgenus Leprocybe species (especially the type of the
subgenus, C.cotoneus Fr. in section Leprocybe, and representatives from the other four
sections) are clearly necessary before a clear circumscription of the subgenus can be made.
|