|
 Austrogaster novae-zelandiae Austrogaster novae-zelandiae
BiostatusPresent in region - Indigenous. Endemic
Images (click to enlarge)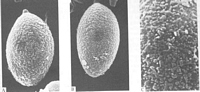
Caption: Fig. 5. SEM studies of spores. A-C. Austrogaster novae-zelandiae (A-B) x19200. (C)
x48000. | 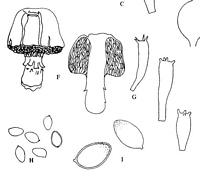
Caption: Fig. 1 F-I. Austrogaster novae-zelandiae (F) Habit sketch x 2/3. (G) Basidia. (H) Spores. (I) Spores x1450. |
Article: Reid, D.A. (1986). New or interesting records of Australasian basidiomycetes: VI. Transactions of the British Mycological Society 86(3): 429-440.
Description: Sporophore up to 6 cm high. Pileus 2.5-3.0 cm high, up to 4 cm wide, more or less conical
with a truncate apex, surface pale ochraceous-brown, formed of radial fibrils, and in places
becoming strongly radially fissured exposing the pale ochraceous flesh. Stipe-columella
continuous, 5.5-6.0 cm high, 1.3-1.5 cm wide at the slightly enlarged base, solid, pale
ochraceous-brown to ochraceous-buff with subfibrillose surface which may disrupt and give
the false impression of a ring-zone. Gleba fulvous, decurrent, tubular-lacunose appearing
irregularly poroid from below, with pores to a mm diam. Cap cuticle of horizontal, hyaline,
undifferentiated repent hyphae comprising two layers - (1) the outermost, which is inclined to
flake away, of more loosely arranged hyphae, 3.0-6.6 µm wide: (2) an inner zone up to 60 µm
thick, of denser agglutinated hyphae a µm wide. This is followed ba the context of broad
inflated hyphae 9.0-16.5 µm wide, with slightly thickened refractive walls and comprising
relatively short segments together with deeply staining, kinked conducting hyphae, 4-5 µm
wide. The hyphae immediately below the two cuticular layers have slightly thicker pale
brownish walls. All hyphae with clamp-connexions. Cystidia not seen. Basidia 33.0-42.0 x
10.0 µm, thin-walled, hyaline, clavate, with 4 sterigmata. Spores ochraceous to fulvous,
9.0-11.2 x 6.0-7.2 µm, elliptic to amygdaliform and with very slightly thickened walls, smooth or
appearing slightly roughened at the apex when examined under oil, non amyloid and with a
centric or lateral apiculus. Under SEM, however, there is a distinct surface ornament.
Notes: Austrogaster has, hitherto, been considered as a South American genus with the two known
species,. A. marthae and A. baeospermum, both described from Argentina. In each of these
taxa the peridium seems to enclose the gleba persistently and completely, unlike the situation
in A. novae-zealandiae where the gleba is exposed below and is irregularly poroid. However,
this new species shares with the other two, certain characters such as hyphae with clamp-connexions and
particularly spores of similar shape and ornamentation. The stipe-columella
is also percurrent in all three species. For these reasons the new taxon has been assigned to
Austrogaster.
A casual glance at A. novae-zelandiae might suggest that it was a species of Gastroboletus,
especially in view of the exposed gleba. However, the type species of this genus, G. boedijnii
as redescribed by Singer (1973), is very different in microscopic structure. Thus the hyphae
lack clamps, there are both pleuro- and cheilocystidia, and the spores are quite unlike.
According to Singer (1973) the latter are `fusoid to cylindric, more or less asymmetrical, with
a deep melleous-brown episporium and a very pale melleous endosporium (both together 1
µm thick), smooth, cyanophilic, with a short hilar appendage which is more often obliquely
than centrally attached, with or without a suprahilar applanation, inamyloid, cyanophilic'.
Clearly in view of these divergent microscopic characters it would be very inappropriate to
assign this new taxon to Gastroboletus.
However, Austrogaster novae-zelandiae does seem to stand in relation to the genus Paxillus,
as Gastroboletus does to Boletus with regard to the degree of Gasteromycetization. In the
other two species of Austrogaster this process seems to have proceeded a stage further, such
that the gleba is persistently enclosed by the peridium.
When Singer (1962) published the genus Austrogaster he assigned it to the family
Gastroboletaceae which he described in the same paper, including also the genera
Truncocolumella, Gautieria, Chamonixia, Brauniellula,
Gastroboletus and with slight doubt
Gymnogaster.
In his account of the spores of the type species of Austrogaster, A. marthae Sing., he wrote:
`Spores (11.3-)12-14.5 x 5.7-6.5 µm ellipsoid, orthotropic or suborthotropic, without a callus
or germ pore, with centrally attached small hilar appendage (straight or slightly oblique), at
first hyaline and smooth, then in young specimens dull melleous and at full maturity most
spores assuming a bright golden melleous color, eventually somewhat thick-walled, almost or
quite smooth and remaining so, some with homogeneous wall even at maturity but some with
heterogeneous wall (this is the majority of the mature spores) i.e. its outermost layer
(episporium) perforated in the manner of Crepidotus spores and finely punctulate when the
surface is focussed upon and the surface appearing very slightly roughened in some spores,
smooth in others; in cresyl blue mounts not metachromatic and ornamentation rather less
distinct, appearing divided in a thin but distinct episporium which is deep colored (also in
iodine solutions), and a hyaline, slightly thicker endosporium, weakly pseudoamyloid when
seen in the Melzer reagent (light rusty cinnamon), which may be a reaction of the episporium
only'.
He also went on to discuss the possible relationships of the new genus and its type species:
`This species is evidently closely related to the paxillaceous Agaricales, particularly Paxillus
sect. Argentini, and at first I was tempted to consider it a gastroid stage or condition of P.
boletinoides which is common all through Patagonia where Nothofagus exists. However, I
convinced myself that this is not the case since in the first place there was no indication that
the peridium ever opens in Austrogaster or ever remains closed in the Boletinus; furthermore,
the faint heterogeneity of the walls of most of the spores of the gastroid species is entirely
missing in all collections of B. boletinoides, and the gastroid configuration of sterigmata and
spores is quite fixedly correlated with Austrogaster exclusively. The characteristic agreeable
odor of drying B. boletinoides is absent in Austrogaster. Nevertheless, the affinity between
the agaricoid and the secotioid group is undeniable. The spore ornamentation in Austrogaster
may indicate a possible relation with the Crepidotaceae also but this cannot be substantiated
by other observations'.
However Singer's interpretation of the spore structure as being heterogeneous with its
outermost layer (episporium) perforated in the manner of Crepidotus spores ... etc. has not
been confirmed by subsequent SEM studies.
Horak & Moser (1965) placed Austrogaster in their new subfamily Paxillogastroideae along
with three new genera which they also described on the same occasion, viz. Singeromyces
Moser, Paxillogaster Horak and Gymnopaxillus Horak. The spore data for the new subfamily
were as described previously by Singer for the family itself. This included the phrase `spores
levibus vel leviusculis (exosporio perforato-punctato)' and indeed in the key to taxa one has
to say that Austrogaster marthae possesses such spores to arrive at this species.
However, because Austrogaster has clamp-bearing hyphae and elongate-ellipsoid spores
Pegler & Young (1981) preferred to regard the genus as a member of Paxillaceae. They
studied the fine structure of the spores of A. marthae and found them to be `Spores 10.5-14.5
x 5-6.5(11.8 ±0.9 x 5.6 ±0.36 µm); Q = 2.0, elongate ellipsoid, either radially symmetrical or
adaxially applanate, at first subhyaline becoming yellowish brown, with a thickened wall
bearing a uniformly verrucose eusporial ornamentation. The ornamentation is often obscured
by a persistent myxosporium which finally fragments and falls away. Although the adaxially
flattened spores appear asymmetric, the ornament is present in the suprahilar region and no
plage area is delimited. A prominent basal hilar appendix bears a large terminal discoid hilum
to which sterigmal fragments are often attached'.
However they noted that while ornamented spores in Paxillaceae are unusual, very similar
spores to those of A. marthae and A. novae-zelandiae occur in Paxillus zerovae from the
Ukraine.
So current opinion has subscribed to Singer's (1962) view that Austrogaster is closely related
to Paxillus even though the spore structure is now known to be slightly different from
Singer's original interpretation.
Another fungus which might be confused with A. novae-zelandiae is Thaxterogaster
epiphaeum Horak, for apart from morphological similarity it too was collected in the same
general area as the holotype of the latter (Cass, Canterbury Province, New Zealand). However, it
would seem that T. epiphaeum tends to be more `elance' with a proportionally longer and
narrower stipe, the gleba is distinctly adnate or adnexed and is covered by a thin white veil. There
are also microscopical differences in that the spores of T. epiphaeum, although of similar size
(9.5-14.0 x 6.0-7.0 µm) to those of A. novae-zelandiae, are elliptic and appear distinctly warted
under oil, especially toward the bluntly rounded apex. In contrast those of Austrogaster novae-zelandiae
(9.0-11.2 x 6.0-7.2 µm) are somewhat amygdaliform and narrow toward the apex; they
are also virtually smooth, showing only the slightest indication of an ornament toward the extreme
apex when viewed under oil. Examination of the spores under SEM has revealed that there is a
very fine ornament over the entire surface, although more prominent over the spore apex; this
ornament is formed by disruption of the eusporium giving an irregular scribbled pattern. In
contrast SEM investigation of the spores of T. epiphaeum shows them to be covered with a
coarse verrucose ornament, also more prominent over the spore apex. Another distinction between
the two fungi involves differences in cuticular structure. In A. novae-zelandiae the cuticle is
two-layered with an outer zone of loosely arranged hyphae and an inner zone of densely
agglutinated narrow hyphae; in T. epiphaeum it comprises an epicutis `of radially arranged,
gelatinized, cylindrical (2.6 µm diam.) hyphae forming a cutis, membrane thin-walled and
encrusted with brown pigment'. However, undue reliance should not be placed on these apparent
differences of cuticular structure as the observations with regard to A. novae-zelandiae were
made on dried material and need to be confirmed from a study of fresh specimens. In summary
there seems to be ample reason to regard the Stevenson collection as representing a new taxon
distinct from T. epiphaeum and not just an atypical or immature condition of the latter.
|