|
 Xylaria castorea Xylaria castorea
BiostatusPresent in region - Indigenous. Endemic
Images (click to enlarge)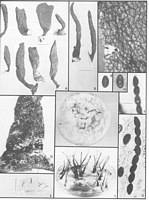
Caption: Fig. 2 Xylaria castorea. A, Stromata (PDD 45771). B, Stromata (PDD 47535). C, Surface
of stroma (PDD 45733). D, Base of stromal stipe (PDD 45733). E. Anamorph from nature
(PDD 47535). F, Ascal ring and ascospores (Melzer's reagent, PDD 45732). | 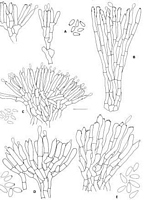
Caption: Fig. 6 Xylaria anamorphs A, B, X. castorea (A, conidiophores and conidia from culture
PDD 44288; B, conidiophores from nature PDD 44280). C, X. cubensis (from culture,
PDD 49005). D, X. tuberiformis (typical | 
Caption: Xylaria castorea, Auckland Islands.
Owner: Peter Johnston | 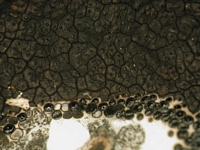
Caption: close up of stroma and section
Owner: J.A. Cooper | 
Caption: fruitbodies
Owner: J.A. Cooper | 
Owner: J.A. Cooper | 
Owner: J.A. Cooper | 
Caption: scale=2mm
Owner: J.A. Cooper | 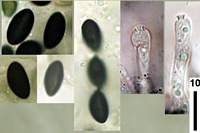
Caption: spores showing faint germ slit and immature asci showing apical apparatus
Owner: J.A. Cooper |
Article: Berkeley, M.J. (1855). Fungi. Flora Novae-Zelandiae. Part II. Flowerless Plants. The Botany of the Antarctic Voyage II Hooker, J.D. (eds.) 378 p. 172-210, 338 London: Lovell Reeve.
Description: Stem about 1/4 of an inch high, at first clothed with spongy down, then naked, longitudinally
wrinkled; head ovate, or subelliptic, strongly compressed, obtuse, about 1 inch long, and ½ to
2/3 broad, sometimes giving off a second head at the base, minutely areolate, dotted with the
slightly prominent ostiola. Asci slender; sporidia subelliptic, 1/2500 of an inch long
Notes: A very distinct species, resembling most X. lingua. Lév. True X polymorpha has the sporidia
about 1/1000 of an inch long. The name indicates the resemblance of the head to a beaver's
tail.
Article: Rogers, J.D.; Samuels, G.J. (1987) [1986]. Ascomycetes of New Zealand 8. Xylaria. New Zealand Journal of Botany 24(4): 615-650 (http://www.rsnz.org/publish/abstracts.php).
Description: Stromata solitary to gregarious but not fasciculate; unbranched, cylindrical, clavate,
lanceolate or broadly spathulate, (1-)3-4(-6) cm long x 3-25 mm wide x 2-5(-10) mm thick,
tip acute to rounded, entire stroma fertile; sessile or seated on a barely differentiated, plane
or slightly enlarged, glabrous or villous stipe to 7 mm long x 3-4 mm wide; brown to dull
blackish brown. Surface splitting into distinct plates, each plate with 1-2 minute papillae;
perithecia completely immersed, 400-500 µm diam. Asci 130-140 µm total length x (5.0-)5.5-7.0 (-8.0) µm,
sporiferous portion (50-)70-85(-95)
µm, cylindrical; apical ring J+, minute, 2-3 µm wide x 1-2.5 µm high; 8-spored, ascospores
uniseriate with overlapping ends. Ascospores (8.0-)9.5-11.0(-14.0) x (4.5-)5.06.0(-6.5) µm; elliptical,
inequilateral to
nearly symmetric, dark brown, nearly opaque, slit obscure, probably full-length, straight,
parallel to long axis of ascospore. Conidiophores in vivo forming a dense, 25-50 µm
deep palisade over the stromal surface;
comprising branched, colourless, smooth conidiophores, each branch terminating in a
conidiogenous cell; conidiogenous cells (9-)11-15(-17) x 2-3 µm, cylindrical;
condiogenous loci terminal, with a 0.5-1.0 µm wide refractive circular scar remaining after
conidial dehiscence. Conidia (5.0-)5.3-7.7(-9.0) x (1.5-)2.0-2.8(-3.5) µm, oblong to
clavate, colourless, smooth; each with a 1 µm wide, refractive or non-refractive, frilled,
basal abscission scar.
CHARACTERISTICS IN CULTURE: Cultures grown 2 weeks at 20°C in diffuse
daylight on OA 3-5 cm diam.; mycelium cottony, white to pale salmon with no black
colouration on the surface of the agar; stromata arising from centre or margin of colony, or
stromata not forming. Stromata 7-20 x 1-3 mm, cylindrical with tip acute or less frequently
spathulate; at first entirely pale salmon, gradually becoming dark below, without distinctive
hairs; most remaining sterile with conidia found only once in culture.
Habitat: HABITAT: On decaying wood of trees; most often on Nothofagus spp. and Weinmannia
racemosa Linn. f., but also on the dicotyledonous trees Beilschmiedia tawa (A. Cunn.)
Kirk, Pittosporum sp., Pseudopanax colensoi (Hook. f.) Philipson, Griselinia littoralis
Raoul and on the gymnosperms Phyllocladus aspleniifolius (Labill.) Hook. f. var. alpinus
(Hook. f.) Keng, Podocarpus hallii Kirk, and Dacrycarpus dacrydioides (A. Rich.) de
Laubenfels.
Distribution: DISTRIBUTION: (numbers of specimens examined in parentheses). NORTH ISLAND:
Northland (2), Coromandel (1), Gisborne (3), Taupo (9), Taranaki (1). SOUTH ISLAND:
Nelson (9), Buller (4), Westland (8), N Canterbury (8), Fiordland (2), Southland (2).
Notes: Xylaria castorea is the most common Xylaria in New Zealand and is usually an
inhabitant of Nothofagus forests. It was originally described as being the shape of a
beaver's tail (Castor) but, in fact, the broadly spathulate form is less frequently found than
the cylindrical form. The species is extremely variable, the most usual forms being
cylindrical to lanceolate with an acute apex. All of these forms feature a distinctive plate-like
cracking of the stromatal surface, a minute apical ascal ring, and very dark ascospores
with a germ slit that is often difficult to discern. Cultures derived from all of these growth
forms are identical.
Martin (1970) cultured South African specimens of what he believed to be X. castorea.
Colonies were described as white; stromata were produced, but conidia were not observed.
Isolates of X. castorea from New Zealand are salmon-coloured, thus leaving some doubt as
to the identity of the South African collections.
Xylaria castorea is strongly allied to X. curta Fr. and X. feejeensis (Berk.) Fr. and, indeed,
further study might show it to be only a form or subtaxon of the latter species. The
anamorph and cultures described for X. curta (Rogers 1983) resemble those of X. castorea.
Cultures of X. feejeensis from France are similar to those of X. castorea described herein
except that the bases of stromata of the former are conspicuously villose and areas of
colonies sometimes darken; conidia have not been observed (J. D. Rogers, unpublished).
X. castorea can be confused with X. cubensis (Mont.) Fr. Stromata of both of these species
are of similar stature and both have ascospores of approximately the same size but these
two species are not closely related (see above and Rogers 1984).
|