|
 Rosellinia radiciperda Rosellinia radiciperda
BiostatusPresent in region - Exotic
Images (click to enlarge)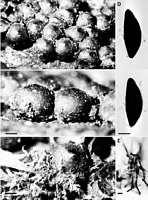
Caption: Fig. 24 Rosellinia radiciperda. A-C, Stromata; D, Ascospores; E, Moss. Type, K. Scale bars:
A = 1 mm; B, C = 0.5 mm; D = 10 µm; E = 20 µm. | 
Caption: Fig. 25 Rosellinia radiciperda. A-D, Stromata, B and C showing subiculum between them; E,
Vertical section of stroma, note stroma shell and detached perithecium; F, Ascospores, | 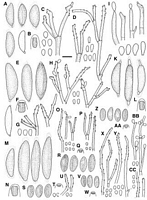
Caption: Fig. 12 A-D, Rosellinia novae-zelandiae, PDD 43205: A, Ascospores, last one immature; B,
Ascus apical ring; C, Conidiophores and conidia on the host (PDD 16422); D, Conidiophores
and conidia in culture (PDD 42074); E-H< |
Article: Petrini, L.E. (2003). Rosellinia and related genera in New Zealand. New Zealand Journal of Botany 41(1): 71-138 (http://www.rsnz.org/publish/abstracts.php).
Description: Subiculum persistent, reduced in mature material, purplish brown, dark brown, woolly to
felty. Stromata (1000)1405 ± 200(2000) µm high, (1125)1628 ± 224(2250) µm wide (n =
155), globose, semiglobose to cupulate, often with a short cylindrical base, copper-brown,
reddish brown, black and shiny around the ostioles, when old dark brown to dull black,
solitary or crowded forming a compact layer, often laterally compressed, when young
completely embedded in the subiculum, only ostioles exposed. Ostioles bluntly rounded or
often not differentiated and then stromatal apex conical. Ectostroma 100-150 µm thick, black,
hard, brittle. Entostroma brown, confined to base, in mature material absent. Perithecia
detached and collapsed in mature material. Ascus apical rings (7.7)10 ± 1.1(12.5) µm high,
upper width 5.7-8.6 µm, lower width 4.8-9.6 µm (n = 52), J+, dark blue. Ascospores
(31.7)39.2 ± 3.2(50) µm long, (6.7)11.9 ± 0.8(16.3) µm wide (n = 460), inequilaterally
ellipsoidal, dark brown, with straight germ slit extending over the whole spore length, both
extremities with one 2-3 x 2-3 µm, semiglobose, cellular appendage, the whole spore
surrounded by a 1 µm thick slimy sheath which is absent in older material. Conidia 6-8 x
3.5-4 µm.
Cultures on OA after 26 days at 20°C under 12 h dark and 12 h UV and fluorescent light
covering plate (9 cm diam.), surface with velutinous black coating of 4-5 µm thick, shortly
branched, melanised hyphae, centre sometimes remaining white, black areas sterile.
Conidiophores variable in length forming at the edge of the plate on plastic or on white
contact mycelium among different isolates, mononematous, mostly dichotomously branched,
smooth, pale tan. Conidiogenous cells 7-51 x 3- 4 µm (n = 21), terminal or less frequently
intercalary,cylindrical, geniculate with conspicuous, circular refractive frill, 1-1.5 µm diam., at
each point of conidial dehiscence. Conidia 5-9 x 2.5-3(4) µm (n = 44), elliptic to oblong,
clavate, with flat, 1-1.5 µm broad base bearing a minute frill, smooth, subhyaline to light tan.
On CMD after 9 days at 20°C under diffused daylight, 2-3 cm diam., flat, white, transparent,
scant aerial mycelium, margin plumelike; after 25 days and under 12 h dark and 12 h UV and
fluorescent light, 4-5 cm diam., grey-brown to dark grey-brown, transparent, no aerial
mycelium, margin feathery, dendroidal. Conidiophores up to 250 µm long, scarce, only at
colony margin, branched once or twice, each branch producing a penicillus of secondary
branches and conidiogenous cells, smooth, pale tan. Conidiogenous cells 8-54 x 3 µm (n =
13), geniculate with prominent circular refractive frill, 1-1.5 µm diam., at each point of
conidial dehiscence. Conidia (4)6-8(9) x 3-4 µm (n = 22), elliptic, with flat, non protuberant,
1-1.5 µm wide base bearing a refractive ring, smooth, subhyaline to pale tan. On PDA after 36
days at 20°C under diffused daylight, rapidly spreading, cottony, with dark brown patches
composed of many shortly branched black hyphae; around the original inoculum velvety, with
erect much-branched hyphae, reverse with some brown discolorations. Areas of conidia
formation grey-tan, at lines of contact between isolates. Conidiophores mononematous, erect,
branched, smooth, hyaline to pale tan.
ANAMORPH: Geniculosporium.
Habitat: HOSTS: Beilschmiedia tarairi, Beilschmiedia tawa, Brachyglottis repanda, Coprosma
australis, Coprosma lucida, Olearia rani, (Pirus malus), Weinmannia sp.,
unidentified hosts.
MATRIX: Corticated, decorticated, heavilydecomposed wood.
Notes: NOTES: Rosellinia radiciperda is easily recognised by the crowded stromata with a blunt
apex and soft slopes, the subiculum with a purplish tinge, and its large, dark brown ascospores
with a long germ slit, two cellular appendages, and slimy sheath.
Saccardo (1899) mentioned a Dematophora anamorph for R. radiciperda. Massee (1896)
described a damaging effect of the mycelial stage of this fungus on its host plants similar to
that known for D. necatrix Hartig. On the North Island only, common orchard trees and also
several native trees and plants, especially growing on the border between indigenous forest
and crop land, were affected. Massee (1896), therefore, postulated that the fungus had to be
native to New Zealand. In fact, recent collections are from the North Island and exclusively on
endemic hosts. He was not able to produce a Dematophora state in his inoculation experiment
with the New Zealand mycelium and soil sample. Later, however, he received a parcel
containing material, also from New Zealand, with fertile stromata collected from the base of a
fallen and decayed Pirus malus. His illustrations of the ascospores match the spore shape of
the type specimen of R. radiciperda. The type is rather old, the subiculum is worn away, and a
dematiaceous hyphomycete and some mosses are present (Fig. 24E). Features of R.
radiciperda are well documented due to the many specimens examined, none of them on
roots. Conidiophores typical of Geniculosporium were once found in the subiculum; in culture
they were also mononematous.
The species most resembling R. radiciperda is R. merrillii, described from the Philippines.
The latter has smaller ascospores and stromata that also lack the bluntly conical apex
characteristic of the New Zealand species.
Article: Dingley, J.M. (1969). Records of plant diseases in New Zealand. New Zealand Department of Scientific and Industrial Research, Bulletin 192: 298 p. Wellington:.
Notes:
Rosellinia radiciperda Massee, Kew Bull. misc.
It is doubtful if this fungus is associated with a root rot; up to the present white root rot in
New Zealand has been recorded under this species. Gilmour (1966a) points out that
cultures of this species are non-pathogenic to Pinus sp. This species needs further
investigation.
|